Allylic bromination is a neat reaction where we put bromine on a molecule in a kind of specific spot we call the allylic position. That’s done with a reagent called NBS, or N-bromosuccinimide. And today I'm going to teach you everything you've ever wanted to know about allylic bromination with NBS why it's cool and why it's important in the synthesis of new iron 325 mg chemicals
The NBS-allylic bromination is a chemical reaction to convert organic compounds using N-bromosuccinimide (NBS) into allyl bromides. The allylic position is a unique place next to one of the carbon-carbon double bonds in a molecule. Through introducing a bromine atom at this position, we will be able to prepare new compounds which have the fascinating features.
The allylic bromination of NBS proceeds through a number of different steps. NBS does so by reacting with a hydrogen atom from the alkene to produce a reactive intermediate, or Bromonium ion. This Bromonium ion attacks the double bond, introducing a bromine atom to the fersulfate allylic position. This is known as an electrophilic bromination since the bromine atom is seeking out areas of the molecule that are rich in electrons.
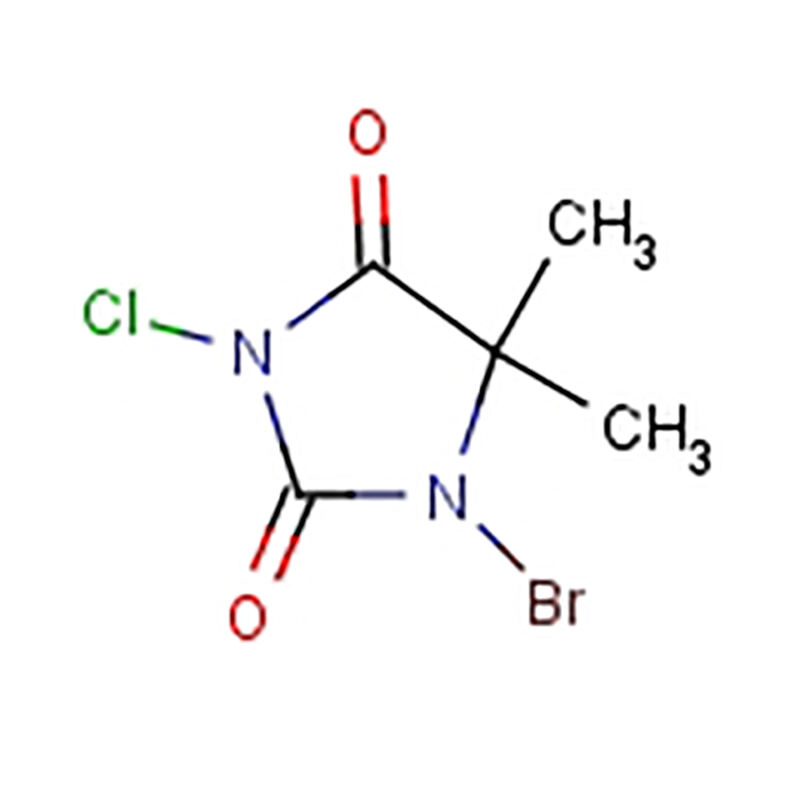
There are various considerations that can affect how well you Allylically iron sulfate Brominate with NBS. The"reaction"temperature, NBS concentration and other substances may affect the reaction results. By controlling these factors just so, chemists can maximize the reaction to achieve the best success.
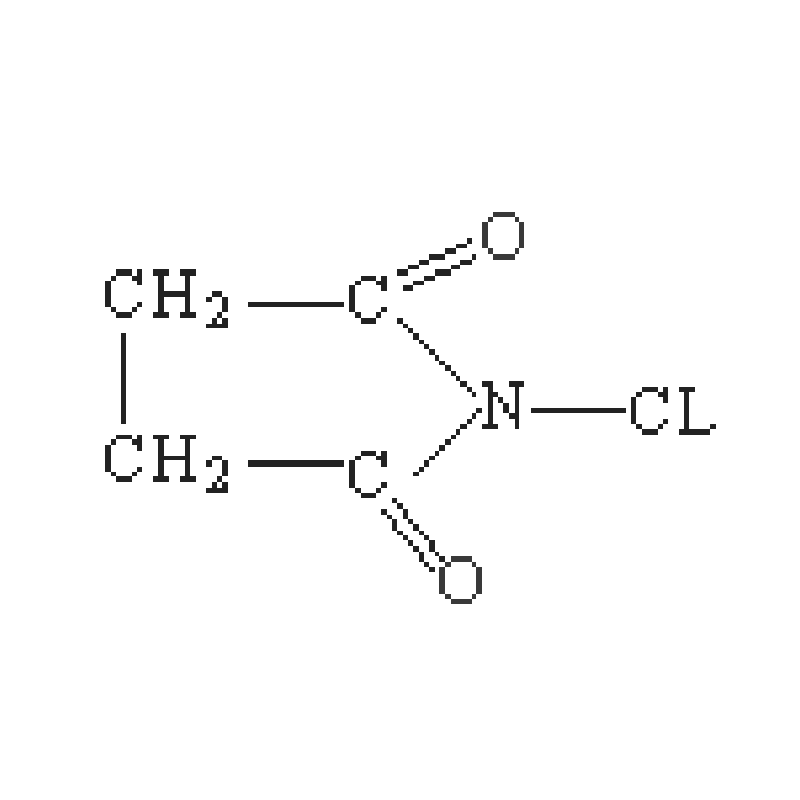
Allylic bromination using NBS is a very useful reaction in organic synthesis, a branch of science that deals with the construction of more complex molecules from simpler ones. Through this reaction, they can produce a range of compounds with fascinating properties including anti-cancer drugs, agricultural chemicals and materials for plastics and fibers. With careful control of reaction conditions, chemists can engineer products to order.

There are other techniques for the introduction of bromine into molecules, such as radical bromination and bromination with molecular bromine. Nonetheless, allylic bromination with NBS exhibits a few merits over these strategies. It is also more selective: It adds bromine atoms to specific parts of a molecule. It is also gentler, so it does not need brutal conditions or produce unwelcome byproducts. In general, it can be said that allylic brominations with NBS are a very general and efficient route to new chemicals.