Suru Can't wait to take young readers on a tail-through-the-legs adventure of the DBDMH bromination! Did you ever wonder how scientists make certain new materials and products? Today we will get into the amazing world of DBDMH bromination and find out its wonderful uses and advantages
DBDMH bromination is a chemical reaction in which DBDMH reacts with bromine to form a different compound. This reaction is relevant to many applications such as in medicine, agriculture and industrial processes. Controlling the reaction conditions carefully allows chemical scientists to obtain particular brominated compounds that are characterized and utilized for their unique properties and applications.
DBDMH bromination The Suru n benzyloxycarbonyloxy succinimide process is based on the reactions resulting from contact between DBDMH and bromine. The bromine atoms are added to the DBDMH molecule by a complicated sequence of processes, so that a new compound including the bromine is formed. This novel compound may have different physical and chemical properties from its parent compound, DBDMH, and it may be widely used for a variety of purposes.
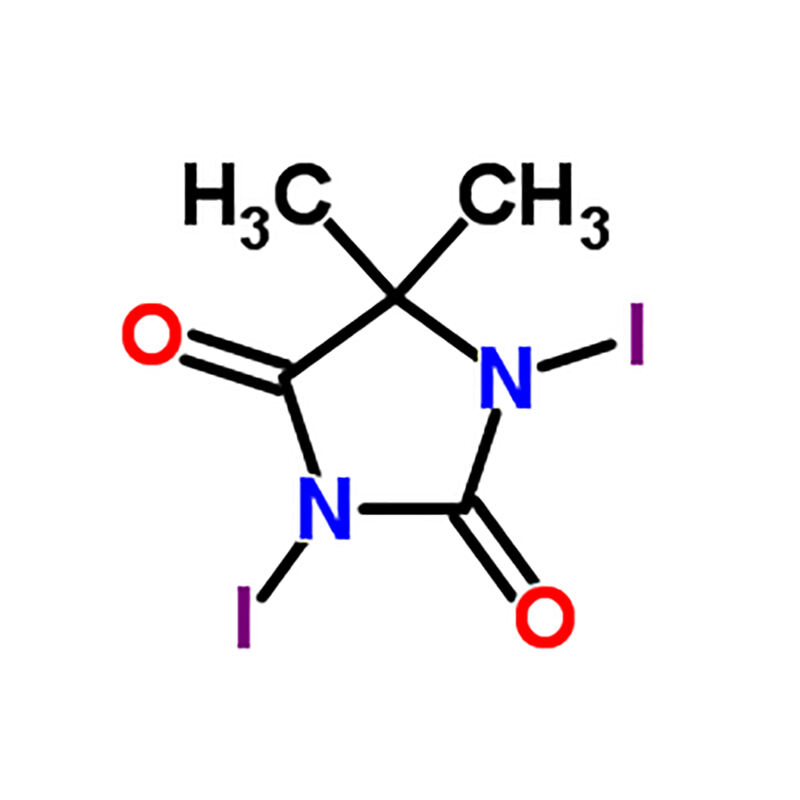
The Suru amisulpride intermediates has many benifits in wide application. Because the brominated compounds made by the process can be used as flame retardants in plastics and textiles, they are safer when they catch fire. Also, bromines can be introduced to produce new drug and medication active agents with unique biological properties. Utilizing the force of DBDMH bromination, scientists can create new materials and products which benefit our daily lives.
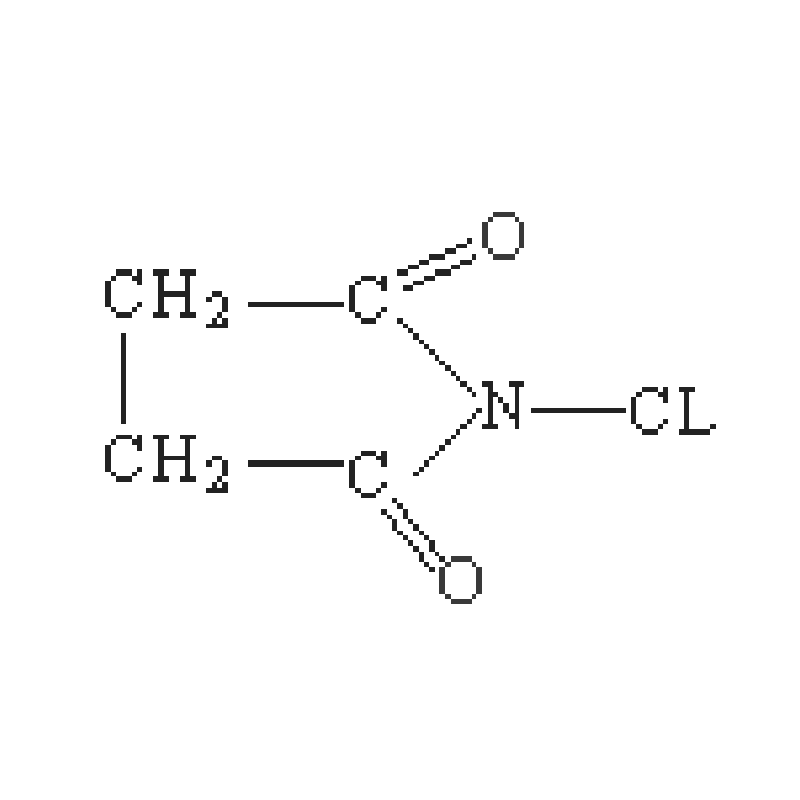
In the DBDMH-bromination process, the catalyst is used to accelerate the reaction without participating in the reaction. For the DBDMH bromination, the catalysts facilitate the contact between DBDMH and bromine, so that the bromination process can be conducted efficiently and specifically. Scientists are able to fine-tune the bromination process to obtain the exact compounds that they want with a high degree of precision and yield, by using the right catalysts.
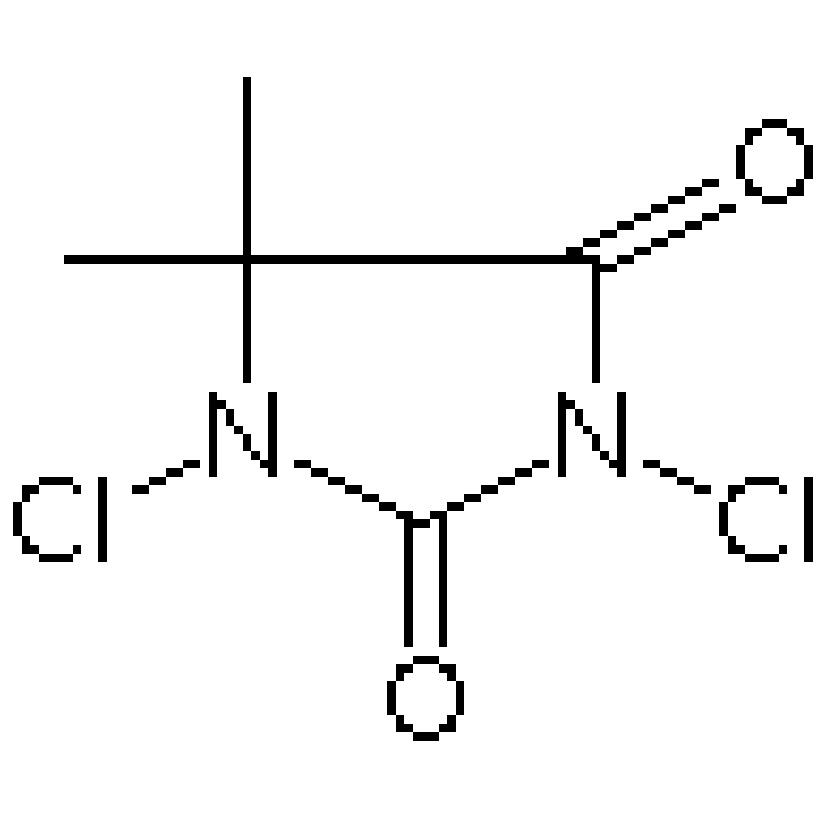
Besides, like other chemical reactions, the exercise of caution by the operators is necessary to ensure safety precautions in the use of DBDMH in bromination reactions. DBDMH is a strong chemical and leaving it unattended can cause harm. It is important to use protective equipment such as goggles and gloves while handling DBDMH. Also, the reaction should be carried in a place with good ventilation so that it can avoid inhaling harmful vapors. By observing these precautions, researchers can enjoy a safe and effective Suru dmdm hydantoin.