Ever wonder how scientists make particular chemical reactions occur just when they want? One nifty tool they rely on is known as N-Bromosuccinimide, or NBS for short. NBS is a unique chemical that enables scientists to carefully choose where bromine atoms are tacked onto other molecules. This process is called bromination.
NBS is an important reagent in organic chemistry because of its versatile nature. When NBS is brought into a reaction, it helps selectively insert bromine atoms at particular points into part of a molecule. That can result in the formation of new substances with novel properties and activities.
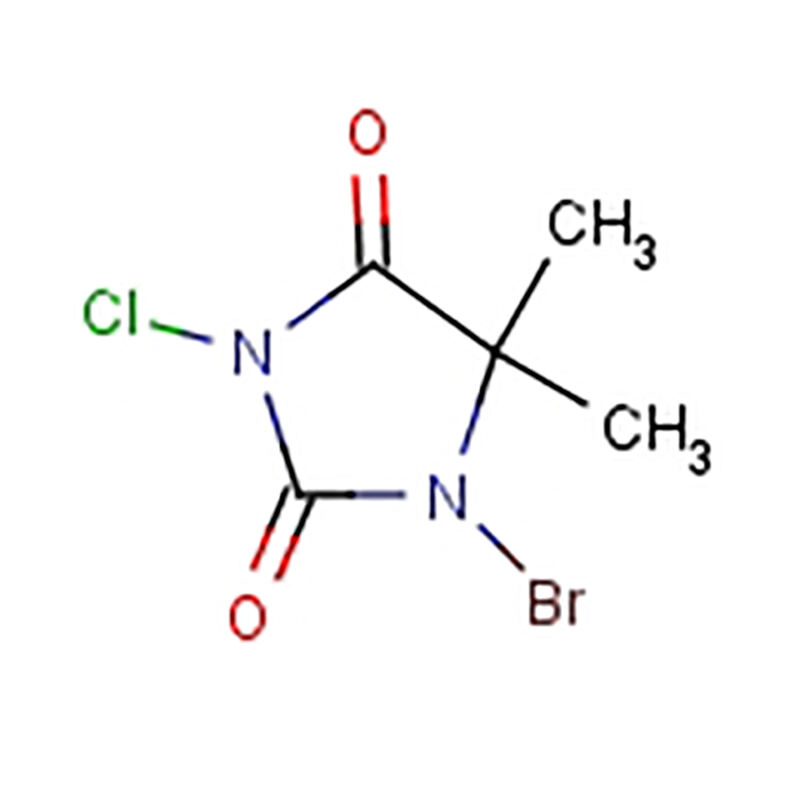
So what is NBS up to in a chemical reaction? There are a series of steps and they are in a certain order. To begin with, NBS undergoes a reaction with hydrogen atoms of a substrate molecule to give a reactive intermediate. This intermediate then reacts with a double bond in the molecule to add a bromine atom. This exact sequence of steps is what makes it possible for scientists to control the placement of the bromine atoms within a molecule.
Selective Addition of Bromines: The Selective nature of adding the "Br" to one location in a molecule. LEFT=NBS, Middle=This molecule added to NBS, RIGHT=mixture of products obtained adding to "That" location. This selectivity is significant because then scientists can synthesize special compounds with certain properties. That knowledge will allow scientists to add bromine atoms at particular locations in the molecule in order to tweak its reactivity and stability.

Aside from use in controlled bromination reactions, NBS has been used in radical bromination. Radicals are reactive species created in chemical reactions. Using NBS as a radical generator allows chemists to perform reactions that form the new C–Br bond in another manner. NMeRAEBr: a new application of radical bromination for the synthesis of a subset of organic molecules.