Nitrobenzene Sulfonate is produced by NBS synthesis. Understanding this process is critical to chemists in creating new compounds. Read on to find out more about how NBS is synthesized and used in (organic) chemistry.
What exactly goes on behind the synthesis of NBS is akin to a secret recipe. Chemists use certain ingredients and steps to produce Nitrobenzene Sulfonate. Effect on settling Most of the reduction in settling imparted by synthetic waxes is due to their co-solubility; that is, ability to react with compounds in the engine oils to produce new products. NBS synthesis is something that chemists study to understand what’s going on and how they can use it in their experiments.
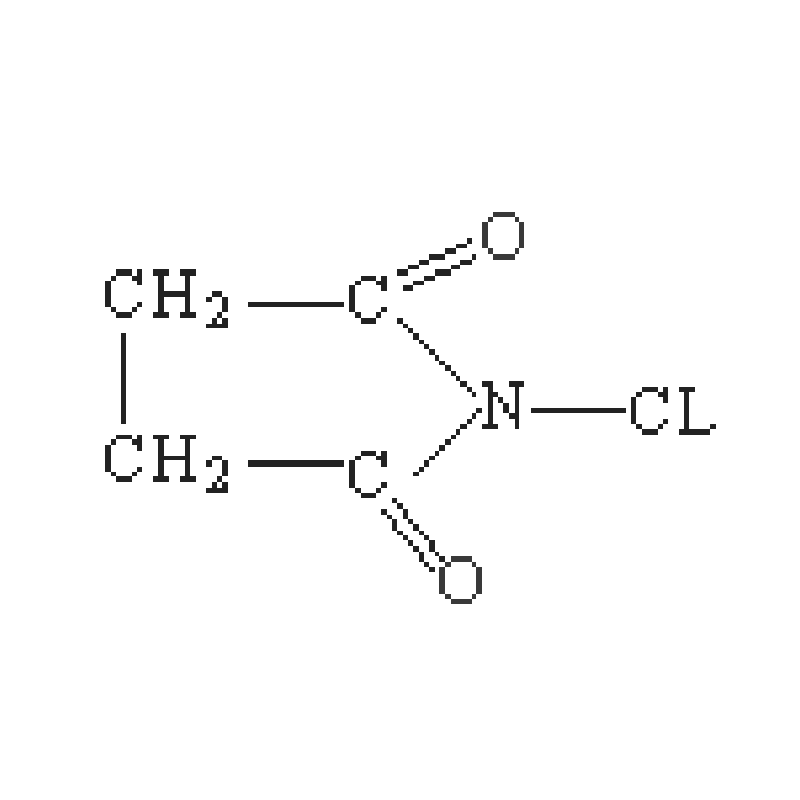
It is not only a NBS synthesis to make Nitrobenzene Sulfonate. It can also be employed to prepare numerous other sulfonate compounds. These compounds have diverse chemical properties and applications. Scientists can produce a host of products that may be used for diverse purposes such as by-products of NBS synthesis. They do this to identify new solutions and discoveries.

NBS synthesis has long been the subject of efforts to make it better and faster. They are telling us how: through R&D. They can improve the efficiency of the NBS synthetic process by experimenting with new methods and technologies. This allows them to produce more Nitrobenzene Sulfonate, in less time and with less waste. Scientists need to keep refining their processes so they can work more efficiently and make new discoveries.

It's synthesis, NBS, is a useful reagent for chemists dealing with aromatic compounds. These are special sorts of molecules which are shaped like a ring. Chemists can alter the properties of aromatic compounds with NBS synthesis. This is how they learn how these things work and how they can be used in different ways. The Description The NBS synthesis is a very handy tool, and it can make the life of an organic chemist synthesising compounds with aromatic rings all the nicer.